Anolyte: powerful biocidal
There are two Anolyte solutions: Acid Anolyte and Neutral Anolyte.
The differences can be assessed as follows:
Active Chlorine, contained in Anolyte, is the biocide/bacterial element. Its concentration is the same in both solutions and the only difference is the pH value. Therefore, in all applications where corrosion is problematic, it is better to use the Neutral Anolyte.
There is also no significant formation of chlorine compounds by organic contact, such as trihalomethanes and there is no toxic by-product of inorganic contact such as chlorites (ClO2) and chlorates (ClO3).
As can be seen from the table, the main compounds that develop within the reactor are hypochloric acid and hypochlorite ion. The percentages are not defined because their sum amounts to 0.05% of the volume, and are linked to the pH value of the solution as can be seen in the following image.
The graph shows a predominance of hypochloric acid (HClO) when the pH values are less than 7.5, while when the pH values are higher there is a predominance of hypochlorite ion (ClO-).
Since the germicidal effect of HClO is much higher (80-120 times) than the germicidal effect of ClO-ions, it is preferable to use low pH chlorination which results in hypochloric acid (HClO) predominance.
In addition to these, very small percentages are also developed: ClO2, HClO3, HClO4, H2O2, O2, ClO2-, ClO3-, O-, HO2-, OH-.
All these components make Neutral Anolyte a powerful bactericide and sporicidal agent.
Cl2(g) + H2O --------> HClO + H+ + Cl-
Hypochloric acid is weak and easily dissociates into hydrogen ions and hypochlorite ions, as can be seen in reaction 2:
HClO <---> H+ + OCl- (2)
When dissolved in water, the following reaction develops:
NaOCl + H2O -------> HClO + Na+ + OH-
Therefore, the addition of hypochlorite sodium to water produces hypochloric acid, similar to the hydrolysis of chlorine gas.
Since the addition of sodium hypochlorite to the water produces hydroxyl ions that increase the pH of the water and any excess sodium hydroxide further increases the pH of the water, the use of sodium hypochlorite for disinfection becomes problematic, also considering the need to use chemical components for both the production and stabilisation of the solution.
The reaction between hypochlorite calcium and water is indicated in the following reaction:
Ca(OCl)2 + 2H2O -------> 2HClO + Ca++ + 2OH-
From the reaction it is noted that the use of calcium hypochlorite in water also produces hypochloric acid, as in the hydrolysis of gaseous chlorine.
Similar to sodium hypochlorite solution, the addition of calcium hypochlorite releases hydroxyl ions that increase the pH of the water.
First of all, being at ph 7 ensures that the ratio of hypochloric acid to hypochlorite ion is clearly shifted towards hypochloric acid, 80% versus 20% hypochlorite ion, ensuring high bactericidal power.
The reactor produces the same biocidal substances (HClO and ClO-) as the other disinfectant agents, but it has many advantages: it is not toxic, neither corrosive nor dangerous, it does not induce resistance in bacteria and eliminates/limits the formation of undesirable substances (THMs, Chlorites, Chlorates,...).
Moreover, imitating the functioning of the immune system, or rather imitating the behaviour of leukocytes (neutrophils, macrophages) is particularly effective.
In fact, the foreign cells are first phagocytized and then destroyed by the action of hypochloric acid (HClO), a biocide agent produced by an enzyme, myeloperoxidase (MPO), starting with hydrogen peroxide (H2O2) and chloride ion Cl-, during the oxidative explosion (respiratory burst).

The differences can be assessed as follows:
| pH | ORP (mv) | Active Chlorine (mg/l) | |
|---|---|---|---|
| Acid Anolyte | 2.0 - 3.5 | 1000-1200 | 500 - 700 |
| Neutral Anolyte | 5.5 - 8.5 | 700 - 900 | 500 - 700 |
There is also no significant formation of chlorine compounds by organic contact, such as trihalomethanes and there is no toxic by-product of inorganic contact such as chlorites (ClO2) and chlorates (ClO3).
Anolyte Composition
| Component | Symbol | Quantity | N° EINECS |
|---|---|---|---|
| Water | H2O | 99.69% | 231-791-2 |
| Sodium chloride | NaCl | 0.26% | 231-598-3 |
| Hypochlorous Acid Hypochlorite ion |
HClO ClO- |
0.05% | 231-959-5 231-668-3 |
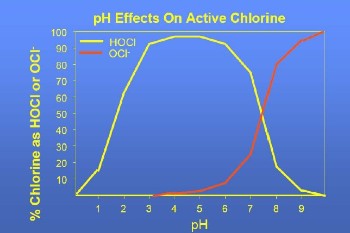
The graph shows a predominance of hypochloric acid (HClO) when the pH values are less than 7.5, while when the pH values are higher there is a predominance of hypochlorite ion (ClO-).
Since the germicidal effect of HClO is much higher (80-120 times) than the germicidal effect of ClO-ions, it is preferable to use low pH chlorination which results in hypochloric acid (HClO) predominance.
In addition to these, very small percentages are also developed: ClO2, HClO3, HClO4, H2O2, O2, ClO2-, ClO3-, O-, HO2-, OH-.
All these components make Neutral Anolyte a powerful bactericide and sporicidal agent.
How do you traditionally obtain Chlorine?
Chlorine Gas
Chlorine gas hydrolyzes rapidly in water to form hypochloric acid (HClO) with the following hydrolysis reaction:Cl2(g) + H2O --------> HClO + H+ + Cl-
Hypochloric acid is weak and easily dissociates into hydrogen ions and hypochlorite ions, as can be seen in reaction 2:
HClO <---> H+ + OCl- (2)
Sodium hypochlorite
Chlorine can also be obtained from sodium hypochlorite, which contains chlorine in a concentration of 12.5%.When dissolved in water, the following reaction develops:
NaOCl + H2O -------> HClO + Na+ + OH-
Therefore, the addition of hypochlorite sodium to water produces hypochloric acid, similar to the hydrolysis of chlorine gas.
Since the addition of sodium hypochlorite to the water produces hydroxyl ions that increase the pH of the water and any excess sodium hydroxide further increases the pH of the water, the use of sodium hypochlorite for disinfection becomes problematic, also considering the need to use chemical components for both the production and stabilisation of the solution.
Calcium hypochlorite
Commercial calcium hypochlorite usually contains 65 percent of available chlorine.The reaction between hypochlorite calcium and water is indicated in the following reaction:
Ca(OCl)2 + 2H2O -------> 2HClO + Ca++ + 2OH-
From the reaction it is noted that the use of calcium hypochlorite in water also produces hypochloric acid, as in the hydrolysis of gaseous chlorine.
Similar to sodium hypochlorite solution, the addition of calcium hypochlorite releases hydroxyl ions that increase the pH of the water.
How can one obtain biological chlorine?
Neutral Anolyte
The neutral Anolyte solution being at pH 7 makes it possible to solve many of the problems related to hypochloric acid generation.First of all, being at ph 7 ensures that the ratio of hypochloric acid to hypochlorite ion is clearly shifted towards hypochloric acid, 80% versus 20% hypochlorite ion, ensuring high bactericidal power.
The reactor produces the same biocidal substances (HClO and ClO-) as the other disinfectant agents, but it has many advantages: it is not toxic, neither corrosive nor dangerous, it does not induce resistance in bacteria and eliminates/limits the formation of undesirable substances (THMs, Chlorites, Chlorates,...).
Moreover, imitating the functioning of the immune system, or rather imitating the behaviour of leukocytes (neutrophils, macrophages) is particularly effective.
In fact, the foreign cells are first phagocytized and then destroyed by the action of hypochloric acid (HClO), a biocide agent produced by an enzyme, myeloperoxidase (MPO), starting with hydrogen peroxide (H2O2) and chloride ion Cl-, during the oxidative explosion (respiratory burst).




