ECA
Electrochemical activation
ECA technology is based on a patented reactor cell, innovative in design compared to traditional electrolytic cells.
As you can see in the picture below,
the fundamental peculiarity of the reactor, is that it allows to separate the two flows of ORPICYL and DETERCYL in a clear way thanks to the presence of a diaphragm in ceramic material that, avoiding the contact between the two solutions, allows only the migration of the ions that are formed thanks to the action of the electric field applied.
As you can see in the picture below,
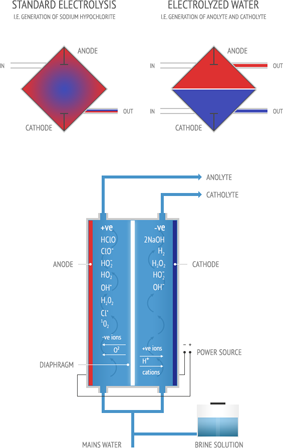
the fundamental peculiarity of the reactor, is that it allows to separate the two flows of ORPICYL and DETERCYL in a clear way thanks to the presence of a diaphragm in ceramic material that, avoiding the contact between the two solutions, allows only the migration of the ions that are formed thanks to the action of the electric field applied.
Reactor performance
In addition to the main electrochemical reaction to electrodes, the high intensity of the electric field on the electrode surface gives an electro-meta-stability degree to water and its dissolved salts, modifying the ORP in the order of -800mv and + 1200mv respectively in the two fluids ORPICYL and DETERCYL.
This meta-stable condition may remain present for periods of up to 72 hours after treatment and even more so if activated solutions are properly stored. In these cases, ORPICYL remains sporicidal for up to 6 months and biocide for up to 18 months.
In addition, the increased ORP value (oxidation reduction potential) improves the solubility and reactivity properties of the water and its dissolved salts to a considerable extent, and is also a significant indicator of the biocidal potential.
Thanks to this conformation, the ORPICYL and DETERCYL solutions are obtainable with a salt consumption (NaCl) that varies according to the size of the cell model.
This conformation, although small in size, allows a production of substance that can vary from 20 liters up to 1200 liters per hour.
Therefore, in the case of dilution at 0.5 ppm, it allows to treat water flow rates from 20 mc/h to 1200 mc/h.
The electrodes, made from a mixture of titanium and rare earths, guarantee high efficiency and low maintenance.
This meta-stable condition may remain present for periods of up to 72 hours after treatment and even more so if activated solutions are properly stored. In these cases, ORPICYL remains sporicidal for up to 6 months and biocide for up to 18 months.
In addition, the increased ORP value (oxidation reduction potential) improves the solubility and reactivity properties of the water and its dissolved salts to a considerable extent, and is also a significant indicator of the biocidal potential.
Thanks to this conformation, the ORPICYL and DETERCYL solutions are obtainable with a salt consumption (NaCl) that varies according to the size of the cell model.
This conformation, although small in size, allows a production of substance that can vary from 20 liters up to 1200 liters per hour.
Therefore, in the case of dilution at 0.5 ppm, it allows to treat water flow rates from 20 mc/h to 1200 mc/h.
The electrodes, made from a mixture of titanium and rare earths, guarantee high efficiency and low maintenance.
Chemistry in the reactor
The solution used in the reactor is essentially water (H2O) and salt (NaCl).
The salt, being soluble in water, decomposes into sodium ions (Na+) and chlorine ions (Cl-).

By applying a voltage between anode and cell cathode and exploiting the permeable properties of the diaphragm against ions, a migration of chlorine ions into the anodic chamber and sodium ions in the cathodic chamber occurs.
. In particular, in the anodic chamber the following reactions take place:
while the following reactions take place in the cathodic chamber:
These reactions give rise to the two activated solutions:

By applying a voltage between anode and cell cathode and exploiting the permeable properties of the diaphragm against ions, a migration of chlorine ions into the anodic chamber and sodium ions in the cathodic chamber occurs.
. In particular, in the anodic chamber the following reactions take place:
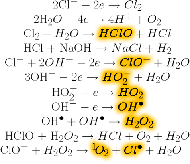
while the following reactions take place in the cathodic chamber:
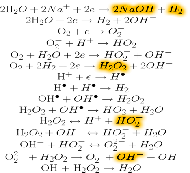
These reactions give rise to the two activated solutions:
Maintenance
The maintenance of the ELA systems produced by Envirolyte depends on the characteristics of the treated water; however, it is extremely reduced, limiting itself to cleaning the cell with Acetic Acid or Chloridic Acid (5-7%) every 800 hours of use.
The time interval can be increased to no less than 2000 hours if the water used has low hardness or is treated with a softener (recommended).
The operating hours are calculated on the basis of the hours in which the unit is producing ORPICYL or DETERCYL; the maintenance intervention is indicated by the PLC, in the most advanced models, based on a measure of the quality of the substance produced.
Why is it useful to understand what is meant by "operating hours" of the unit? Because every litre of ORPICYL produced during operation contains 500ppm of active chlorine which is then diluted in the water you want to treat or in the water that is used to disinfect rooms in different percentages according to the needs of the case. For example:
The time interval can be increased to no less than 2000 hours if the water used has low hardness or is treated with a softener (recommended).
The operating hours are calculated on the basis of the hours in which the unit is producing ORPICYL or DETERCYL; the maintenance intervention is indicated by the PLC, in the most advanced models, based on a measure of the quality of the substance produced.
Why is it useful to understand what is meant by "operating hours" of the unit? Because every litre of ORPICYL produced during operation contains 500ppm of active chlorine which is then diluted in the water you want to treat or in the water that is used to disinfect rooms in different percentages according to the needs of the case. For example:
- - si se va a utilizar agua potable, 1 litro de ORPICYL se diluye en 1 metro cúbico de agua (0,1% de dilución, 0,5 ppm por litro de agua tratada);
- - Si se va a tratar una desinfección estable, 1 litro de anólito se diluye en 50 litros de agua (dilución al 2%, 10 ppm por litro de agua utilizado para desinfectar).
Operating costs
Operating costs depend on the type of ECA unit you are using. There are three ECA unit families:
These consumptions are lower than those found with the other units; in particular about 5 times compared to EL units and about 2.5 times compared to ELA units;
. The units built for the production of ORPICYL also have a small production of DETERCYL, which can be estimated at about 0.4% of the volume of ORPICYL product.
There are also particular units for the production of large volumes of DETERCYL and in this case, the waste is the ORPICYL always with percentages equal to 0.4% of the volume of DETERCYL produced.
In addition, there are also units that are often used for disinfection of food processinf plants, breweries and beverage facilities that allow the production of both liquids. In particular, they can produce ORPICYL with pH between 6.0 and 7.0 and FAC (active chlorine) equal to 500ppm, while DETERCYL will have pH between 12.2 and 12.5 and NaOH concentration between 08-1 gr/L.
In this case, the salt consumption would be 1.5 g per litre of ORPICYL produced, and 3.8 g per litre of DETERCYL produced.
- - EL Unit: Fully manual unit, less expensive to purchase but requires the presence of the operator for its operation;
- - ELA Unit: semi-automatic unit managed through a PLC that informs of the maintenance status. It is semi-automatic in requires the presence of an operator for cleaning manoeuvres during the washing cycle;
- - ELA ANW Unit: is a unit similar to ELA but optimized to reduce energy and salt consumption.
These consumptions are lower than those found with the other units; in particular about 5 times compared to EL units and about 2.5 times compared to ELA units;
. The units built for the production of ORPICYL also have a small production of DETERCYL, which can be estimated at about 0.4% of the volume of ORPICYL product.
There are also particular units for the production of large volumes of DETERCYL and in this case, the waste is the ORPICYL always with percentages equal to 0.4% of the volume of DETERCYL produced.
In addition, there are also units that are often used for disinfection of food processinf plants, breweries and beverage facilities that allow the production of both liquids. In particular, they can produce ORPICYL with pH between 6.0 and 7.0 and FAC (active chlorine) equal to 500ppm, while DETERCYL will have pH between 12.2 and 12.5 and NaOH concentration between 08-1 gr/L.
In this case, the salt consumption would be 1.5 g per litre of ORPICYL produced, and 3.8 g per litre of DETERCYL produced.



